
Chemistry, 30.06.2019 21:30 jackie0833
1. a balloon shrinks when it ’s taken outside in the winter. 2. when the size of an air chamber is increased, the air pressure decreases. 3. a balloon expands when air is blown into it. 4. a closed, flexible container expands when it’s heated. 5. pressing on an inflated balloon decreases its size. match each scenario to the law that explains it. charles's law boyle's law avogadro's law

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
1. a balloon shrinks when it ’s taken outside in the winter. 2. when the size of an air chamber is i...
Questions


Mathematics, 10.06.2021 01:20




English, 10.06.2021 01:20


Mathematics, 10.06.2021 01:20

Mathematics, 10.06.2021 01:20








Mathematics, 10.06.2021 01:20



Mathematics, 10.06.2021 01:20

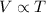 (At constant Pressure and number of moles)
(At constant Pressure and number of moles)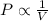 (At constant Temperature and number of moles)
(At constant Temperature and number of moles)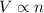 (At constant Pressure and Temperature)
(At constant Pressure and Temperature)

