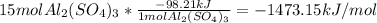Chemistry, 30.06.2019 19:00 lilchannelll4125
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance the reaction. calculate the total enthalpy change that would occur from 15 moles of al2(so4)3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance...
Questions

Mathematics, 31.07.2019 14:00

French, 31.07.2019 14:00

Health, 31.07.2019 14:00

History, 31.07.2019 14:00

Mathematics, 31.07.2019 14:00





Mathematics, 31.07.2019 14:00

Health, 31.07.2019 14:00



Physics, 31.07.2019 14:00

Computers and Technology, 31.07.2019 14:00




History, 31.07.2019 14:00



![H_{reaction}^{0}=[H_{f}^{0}(Al_{2}O_{3}(s)) + (3*H_{f}^{0}(H_{2}SO_{4}(aq))] - [H_{f}^{0}(Al_{2}SO_{4}(aq)) + (3*H_{f}^{0}(H_{2}O(l))]](/tpl/images/0035/8926/80689.png)
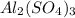 reacts will be=
reacts will be=