Chemistry, 30.06.2019 18:30 serenityjohnson98765
Achemist prepares two solutions: one from 28 g potassium bromide (kbr) and 1 kg water; and the other from 28 g sodium iodide (nai) and 1 kg water. what information is most useful to determine the solution that has the lower freezing point? the density of each solution in grams per liter the crystal structures of kbr and nai the molar masses of kbr and nai the molar mass of water

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

You know the right answer?
Achemist prepares two solutions: one from 28 g potassium bromide (kbr) and 1 kg water; and the oth...
Questions

Mathematics, 12.12.2020 16:30



Mathematics, 12.12.2020 16:30

Arts, 12.12.2020 16:30


History, 12.12.2020 16:30

Arts, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30


History, 12.12.2020 16:30




Social Studies, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30


Health, 12.12.2020 16:30

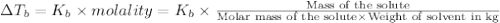
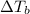 is inversely related to molar mass of the compound. Lower the value of molar mass more will be the value of
is inversely related to molar mass of the compound. Lower the value of molar mass more will be the value of 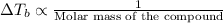
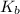 for water is = 0.59 K kg/mol
for water is = 0.59 K kg/mol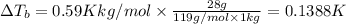
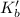 for water is = 0.59 K kg/mol
for water is = 0.59 K kg/mol