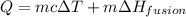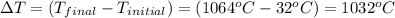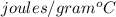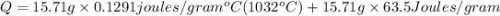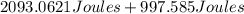The temperature of 15.71 grams of gold rises from 32°c to 1,064°c, and then the gold melts completely. if gold’s specific heat is 0.1291 joules/gram degree celsius and its heat of fusion is 63.5 joules/gram, how much energy is gained by the gold? the gold gained a total of joules of energy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
The temperature of 15.71 grams of gold rises from 32°c to 1,064°c, and then the gold melts completel...
Questions

Mathematics, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00


History, 28.06.2019 11:00

English, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00


Mathematics, 28.06.2019 11:00

History, 28.06.2019 11:00

Chemistry, 28.06.2019 11:00




Mathematics, 28.06.2019 11:00


History, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00