

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
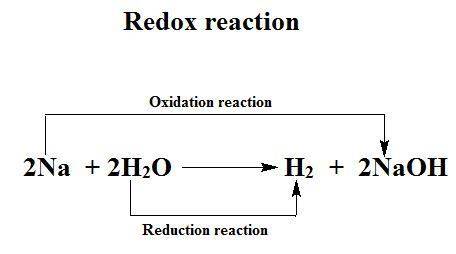
What elements are being reduced and oxidized in this chemical formula? 2na(s) + 2h[tex]_{2}[/tex]o(l...
Questions


Mathematics, 07.02.2021 20:50




Mathematics, 07.02.2021 20:50


Biology, 07.02.2021 20:50



Mathematics, 07.02.2021 20:50


Mathematics, 07.02.2021 20:50






Law, 07.02.2021 20:50

Mathematics, 07.02.2021 20:50

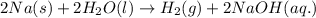
 o(l" />
o(l" />

