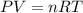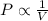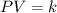Chemistry, 30.06.2019 14:30 YARETZYVENCES2144
Held constant, the pressure and volume of gas . its from boyle's law

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Held constant, the pressure and volume of gas . its from boyle's law...
Questions

Physics, 17.02.2021 06:50

Computers and Technology, 17.02.2021 06:50

Mathematics, 17.02.2021 06:50


Mathematics, 17.02.2021 06:50

Biology, 17.02.2021 06:50

Chemistry, 17.02.2021 06:50

Mathematics, 17.02.2021 06:50

German, 17.02.2021 06:50





Mathematics, 17.02.2021 06:50

Mathematics, 17.02.2021 06:50

Mathematics, 17.02.2021 06:50

History, 17.02.2021 06:50

Mathematics, 17.02.2021 06:50