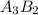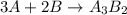Chemistry, 30.06.2019 14:30 sashajayne8260
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a charge of -3. therefore, you determine that the ionic compound (ab) will have a ratio of 3 as for every 2 bs. how would you modify ab in order to show that they have this 3-to-2 ratio? question 2 options: 3a2b a3b2 a3b2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
You are balancing an ionic compound. let's call it: ab. ion a has a charge of +2 and ion b has a c...
Questions

Mathematics, 25.05.2021 19:40


Chemistry, 25.05.2021 19:40


English, 25.05.2021 19:40

Mathematics, 25.05.2021 19:40

Spanish, 25.05.2021 19:40


Physics, 25.05.2021 19:40

Mathematics, 25.05.2021 19:40

Mathematics, 25.05.2021 19:40

Arts, 25.05.2021 19:40

Mathematics, 25.05.2021 19:40


Mathematics, 25.05.2021 19:40

History, 25.05.2021 19:40

Health, 25.05.2021 19:40


History, 25.05.2021 19:40

English, 25.05.2021 19:40