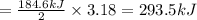Chemistry, 30.06.2019 14:00 ggdvj9gggsc
How much heat is released during the formation of 3.18 mol hcl(g) in this reaction: h2(g)+cl2(g) → 2hcl(g) with a h of -184.6 kj. express your answer in kj.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
How much heat is released during the formation of 3.18 mol hcl(g) in this reaction: h2(g)+cl2(g) →...
Questions

Spanish, 08.04.2021 07:40


Social Studies, 08.04.2021 07:40

English, 08.04.2021 07:40



Mathematics, 08.04.2021 07:40

Mathematics, 08.04.2021 07:40

Mathematics, 08.04.2021 07:40

Mathematics, 08.04.2021 07:40

Social Studies, 08.04.2021 07:40


History, 08.04.2021 07:40






Physics, 08.04.2021 07:40

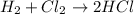
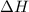 of the reaction is negative, it means the heat is released and the reaction is exothermic.
of the reaction is negative, it means the heat is released and the reaction is exothermic.