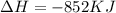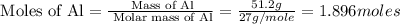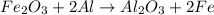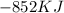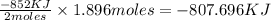Chemistry, 30.06.2019 11:30 nonjabulomabaso6850
How much energy is evolved during the reaction of 51.2 g of al, according to the reaction below? assume that there is excess fe2o3. fe2o3(s) + 2 al(s) â al2o3(s) + 2 fe(s) î´hâ°rxn = -852 kj?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
How much energy is evolved during the reaction of 51.2 g of al, according to the reaction below? as...
Questions

Mathematics, 29.06.2020 02:01

Mathematics, 29.06.2020 02:01

Mathematics, 29.06.2020 02:01

Mathematics, 29.06.2020 02:01



Spanish, 29.06.2020 02:01

Mathematics, 29.06.2020 02:01


English, 29.06.2020 02:01

Health, 29.06.2020 02:01

Physics, 29.06.2020 02:01






Mathematics, 29.06.2020 02:01


Mathematics, 29.06.2020 02:01

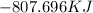 .
.