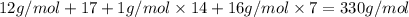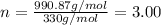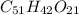Chemistry, 30.06.2019 11:30 megandalolipop
Aformer high school teacher was discovered making meth, and the dea recently analyzed some confiscated material from a drug bust he was associated with. they found a compound with the empirical formula c17h14o7 that has a molar mass of 990.87 g/mol. what is the value of n (the multiplier) necessary to find the molecular formula?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

You know the right answer?
Aformer high school teacher was discovered making meth, and the dea recently analyzed some confiscat...
Questions

Advanced Placement (AP), 01.04.2021 22:00



Mathematics, 01.04.2021 22:00

Mathematics, 01.04.2021 22:00

Mathematics, 01.04.2021 22:00

English, 01.04.2021 22:00

Mathematics, 01.04.2021 22:00



Mathematics, 01.04.2021 22:00


Computers and Technology, 01.04.2021 22:00




Mathematics, 01.04.2021 22:00



Law, 01.04.2021 22:00

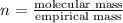
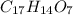 :
: