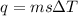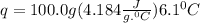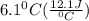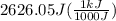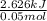A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter. cuso4 (1m) + 2koh (2m) cu(oh)2(s) + k2so4 (0.5m). the temperature of both solutions was 20.2 â°c before mixing and 26.3 â°c after mixing. the heat capacity of the calorimeter is 12.1 j/â°c. assume the specific heat and density of the solution after mixing are the same as those of pure water. from this data, calculate theî´h for the process if there is 0.05 mols of cuso4. (energy of the water + energy of the calorimeter)/(1000 x mol)= kj/mol of reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter....
Questions

English, 14.04.2021 22:00


Geography, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00





German, 14.04.2021 22:00


Mathematics, 14.04.2021 22:00


Social Studies, 14.04.2021 22:00



Social Studies, 14.04.2021 22:00

Spanish, 14.04.2021 22:00



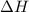 = -
= -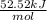
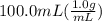
 = 26.3 - 20.2 = 6.1 degree C
= 26.3 - 20.2 = 6.1 degree C