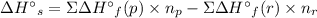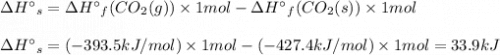Chemistry, 30.06.2019 07:00 ineedhelp2285
Use standard enthalpies of formation to calculate the change in enthalpy for dry ice sublimation. (the î´hâf for co2(s) is - 427.4kj/mol).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Use standard enthalpies of formation to calculate the change in enthalpy for dry ice sublimation. (t...
Questions


History, 10.12.2021 18:30


Mathematics, 10.12.2021 18:30




Biology, 10.12.2021 18:30





Mathematics, 10.12.2021 18:30




History, 10.12.2021 18:30

Mathematics, 10.12.2021 18:30


English, 10.12.2021 18:30