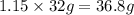Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Given the following equation: 2h2o --> 2h2 +o2 what mass of oxygen would form from 2.30 moles o...
Questions

French, 24.11.2021 15:40

Biology, 24.11.2021 15:40



English, 24.11.2021 15:40

Mathematics, 24.11.2021 15:40







Physics, 24.11.2021 15:40


Geography, 24.11.2021 15:50




Social Studies, 24.11.2021 15:50

Arts, 24.11.2021 15:50

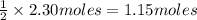
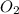 is 32 g/mol
is 32 g/mol