
Chemistry, 30.06.2019 02:30 brooke3493
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, the reaction is said to be endothermic and â†h is negative (–). c) bond dissociation energies are always positive numbers. d) the stronger the bond, the lower its bond dissociation energy. 2. based on the reaction, n2 + o2 → 2 no â†h = 43.2 kcal which statement is true? a) 43.2 kcal are consumed when 14.00 g of n2 reacts. b) 43.2 kcal are produced when 1.00 g of o2 reacts. c) 43.2 kcal are produced when 1.00 mole of o2 reacts. d) 43.2 kcal are consumed when 2.00 moles of no is produced.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, t...
Questions




Mathematics, 23.10.2020 18:10


Mathematics, 23.10.2020 18:10

English, 23.10.2020 18:10



Physics, 23.10.2020 18:10


Mathematics, 23.10.2020 18:10

Mathematics, 23.10.2020 18:10

Mathematics, 23.10.2020 18:10

Mathematics, 23.10.2020 18:10

Mathematics, 23.10.2020 18:10




Computers and Technology, 23.10.2020 18:10

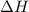 will be positive not negative.
will be positive not negative.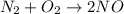
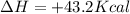
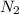 is reacted.
is reacted.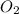 is reacted.
is reacted.

