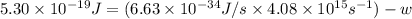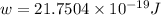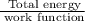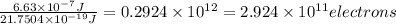Chemistry, 30.06.2019 00:30 lilmamagodchild123
When a metal was exposed to photons at a frequency of 4.08× 1015 s–1, electrons were emitted with a maximum kinetic energy of 5.30× 10–19 j. calculate the work function of this metal. also, what is the maximum number of electrons that could be ejected from this metal by a burst of photons (at some other frequency) with a total energy of 6.36× 10–7 j? i would love an explanation if that is possible!

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
When a metal was exposed to photons at a frequency of 4.08× 1015 s–1, electrons were emitted with a...
Questions



Mathematics, 09.09.2019 18:30



Mathematics, 09.09.2019 18:30


Social Studies, 09.09.2019 18:30

Social Studies, 09.09.2019 18:30


English, 09.09.2019 18:30




Geography, 09.09.2019 18:30


Mathematics, 09.09.2019 18:30


Mathematics, 09.09.2019 18:30


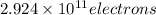
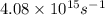
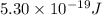
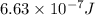
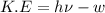
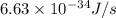
 = frequency
= frequency