
Chemistry, 30.06.2019 00:00 brebun4742
Which two electron configurations represent elements that would have similar chemical properties? (1) 1s22s22p4 (2) 1s22s22p5 (3) [ar]4s23d5 (4) [ar]4s23d104p5?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Which two electron configurations represent elements that would have similar chemical properties? (...
Questions

History, 07.02.2021 23:10



English, 07.02.2021 23:10







English, 07.02.2021 23:10


Physics, 07.02.2021 23:10

Chemistry, 07.02.2021 23:10





Mathematics, 07.02.2021 23:10

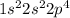
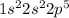
![[Ar]4s^23d^5](/tpl/images/0032/8459/ef380.png)
![[Ar]4s^23d^{10}^4p^5](/tpl/images/0032/8459/97005.png)


