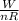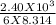Chemistry, 30.06.2019 00:00 scottbrandon653
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial temperature of the gas is 27.0c and the pressure is constant. as part of a machine design project, calculate the final temperature of the gas after it has done 2.40 * 103 j of work.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial tem...
Questions





Mathematics, 17.12.2020 09:40


Mathematics, 17.12.2020 09:40


History, 17.12.2020 09:40


Biology, 17.12.2020 09:40

SAT, 17.12.2020 09:40

Mathematics, 17.12.2020 09:40

Chemistry, 17.12.2020 09:40


Advanced Placement (AP), 17.12.2020 09:40


English, 17.12.2020 09:40