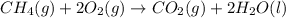Chemistry, 30.06.2019 00:00 alexwlodko
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9 mol of the methane gas. the standard molar enthalpy of combustion of methane is 890.2kj/mol. answer in units of kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9...
Questions

Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

English, 12.12.2020 16:10




Mathematics, 12.12.2020 16:10




Social Studies, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

History, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10