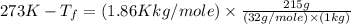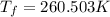Chemistry, 29.06.2019 23:30 FunnySkittle
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is the freezing point of this solution? [the freezing point depression constant for water is 1.86°c/mole solute in 1000g of water]

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is t...
Questions


Mathematics, 04.02.2020 03:43






History, 04.02.2020 03:43





Mathematics, 04.02.2020 03:43







Mathematics, 04.02.2020 03:43

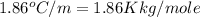
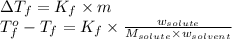
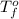 = freezing point of water =
= freezing point of water = 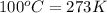
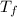 = freezing point of solution
= freezing point of solution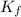 = freezing point constant
= freezing point constant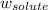 = mass of solute
= mass of solute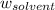 = mass of solvent
= mass of solvent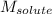 = molar mass of solute
= molar mass of solute