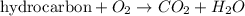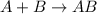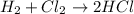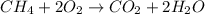Chemistry, 29.06.2019 22:30 strodersage
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents , and equation b represents . blank 1: combustion decomposition double displacement single displacement synthesis blank 2: combustion decomposition double displacement single displacement synthesis

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions

Mathematics, 12.04.2021 19:30


Mathematics, 12.04.2021 19:30



Mathematics, 12.04.2021 19:30

Computers and Technology, 12.04.2021 19:30

Mathematics, 12.04.2021 19:30

Mathematics, 12.04.2021 19:30

Mathematics, 12.04.2021 19:30

Mathematics, 12.04.2021 19:30

Biology, 12.04.2021 19:30







Mathematics, 12.04.2021 19:30