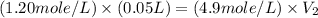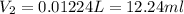Chemistry, 29.06.2019 22:00 claytonp7695
Astudent needs to prepare 50.0 ml of a 1.20 m aqueous h2o2 solution. calculate the volume of 4.9 m h2o2 stock solution that should be used to prepare the solution.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Astudent needs to prepare 50.0 ml of a 1.20 m aqueous h2o2 solution. calculate the volume of 4.9 m h...
Questions

Geography, 20.09.2020 05:01

History, 20.09.2020 05:01

World Languages, 20.09.2020 05:01

Chemistry, 20.09.2020 05:01

Spanish, 20.09.2020 05:01




Mathematics, 20.09.2020 05:01

Spanish, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01



Mathematics, 20.09.2020 05:01

History, 20.09.2020 05:01

History, 20.09.2020 05:01


Geography, 20.09.2020 05:01


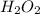 stock solution used to prepare the solution is, 12.24 ml
stock solution used to prepare the solution is, 12.24 ml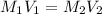
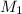 = Molarity of aqueous
= Molarity of aqueous 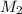 = Molarity of
= Molarity of 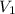 = Volume of aqueous
= Volume of aqueous 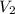 = Volume of
= Volume of