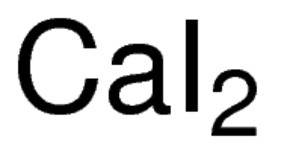Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Calcium forms ions with a charge of +2. iodine forms ions with a charge of -1. which of the followin...
Questions




Mathematics, 26.11.2019 08:31


Mathematics, 26.11.2019 08:31


Health, 26.11.2019 08:31



Biology, 26.11.2019 08:31

Mathematics, 26.11.2019 08:31

Mathematics, 26.11.2019 08:31


Mathematics, 26.11.2019 08:31



Mathematics, 26.11.2019 08:31

Chemistry, 26.11.2019 08:31