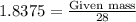The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 92.00 g of si and 112.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions

Mathematics, 28.08.2020 05:01

Social Studies, 28.08.2020 05:01


Mathematics, 28.08.2020 05:01

Business, 28.08.2020 05:01

Chemistry, 28.08.2020 05:01

Mathematics, 28.08.2020 05:01


English, 28.08.2020 05:01

Mathematics, 28.08.2020 05:01

Engineering, 28.08.2020 05:01




English, 28.08.2020 05:01


History, 28.08.2020 05:01



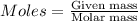 ....(1)
....(1)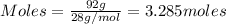
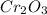
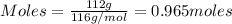
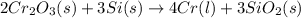
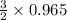 = 1.4475 moles of Silicon.
= 1.4475 moles of Silicon.