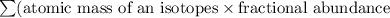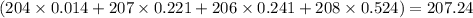Chemistry, 29.06.2019 15:00 webbjalia04
There are four isotopes of lead and their relative abundance listed below. use this information to calculate the approximate atomic mass of lead. show all work (4 points) 82 protons, 122 neutrons, 1.4% 82 protons, 125 neutrons, 22.1% 82 protons, 124 neutrons, 24.1% 82 protons, 126 neutrons, 52.4% show !

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
There are four isotopes of lead and their relative abundance listed below. use this informati...
Questions

Biology, 04.03.2021 01:00

Chemistry, 04.03.2021 01:00

Mathematics, 04.03.2021 01:00

Mathematics, 04.03.2021 01:00

Mathematics, 04.03.2021 01:00


Mathematics, 04.03.2021 01:00

Mathematics, 04.03.2021 01:00






Biology, 04.03.2021 01:00

Physics, 04.03.2021 01:00




Health, 04.03.2021 01:00