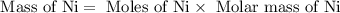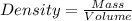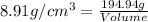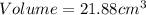Chemistry, 29.06.2019 14:30 school4life110
27. the density of nickel is 8.91 g/cm3. how large a cube, in cm3, would contain 2.00 x 10^24 atoms of nickel? use dimensional analysis to solve and show all work including units on every number! (5 points)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
27. the density of nickel is 8.91 g/cm3. how large a cube, in cm3, would contain 2.00 x 10^24 atoms...
Questions



Business, 13.04.2021 21:30



Arts, 13.04.2021 21:40



Mathematics, 13.04.2021 21:40


Mathematics, 13.04.2021 21:40

Social Studies, 13.04.2021 21:40




Social Studies, 13.04.2021 21:40




Medicine, 13.04.2021 21:40

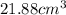
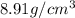
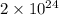
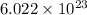 atoms form 1 mole of nickel
atoms form 1 mole of nickel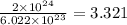 moles of nickel
moles of nickel