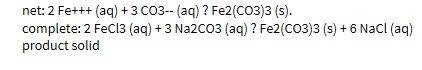Chemistry, 29.06.2019 14:30 brodysalander007
32. write the net ionic equation for the reaction of sodium phosphate with iron (iii) chloride. include the complete ionic equation and label the spectator ions. also be sure to label the reactants as products as solid, liquid, gas, or aqueous solutions. (5 points)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
32. write the net ionic equation for the reaction of sodium phosphate with iron (iii) chloride. incl...
Questions

Mathematics, 09.11.2020 20:10


Mathematics, 09.11.2020 20:10






Mathematics, 09.11.2020 20:10

Spanish, 09.11.2020 20:10




Arts, 09.11.2020 20:10





Mathematics, 09.11.2020 20:10

Mathematics, 09.11.2020 20:10