
Chemistry, 29.06.2019 08:30 Calumworthy6046
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k and total pressure 1.00 bar. calculate the amounts of the components in the mixture at equilibrium given that k = 870 for the reaction h2 (g) + i2 (g) ⇜ 2 hi (g).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k an...
Questions

Mathematics, 14.07.2019 06:30


Mathematics, 14.07.2019 06:30



Health, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30



Mathematics, 14.07.2019 06:30


Mathematics, 14.07.2019 06:30

History, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30




Physics, 14.07.2019 06:30

Mathematics, 14.07.2019 06:30

![[HI] _{eq}=0.825mol](/tpl/images/0030/3484/a93b2.png)
![[H_2] _{eq}=0.010mol](/tpl/images/0030/3484/29662.png)
![[I_2] _{eq}=0.078mol](/tpl/images/0030/3484/3a542.png)
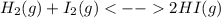
 ):
):![\frac{Kp}{(RT)^{2-2} }=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}} \\Kp=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}}](/tpl/images/0030/3484/98372.png)
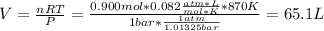
![[H_2] _0=\frac{0.300mol}{65.1L}=0.005M](/tpl/images/0030/3484/0ef57.png)
![[I_2] _0=\frac{0.400mol}{65.1L}=0.006M](/tpl/images/0030/3484/d9a8e.png)
![[HI] _0=\frac{0.200mol}{65.1L}=0.003M](/tpl/images/0030/3484/e3fd2.png)
 due to the equilibrium:
due to the equilibrium: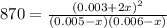
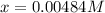
![[HI] _{eq} =(0.003M +2(0.00484M))*65.1L=0.825mol](/tpl/images/0030/3484/1d681.png)
![[H_2] _{eq} =(0.005M -0.00484M)*65.1L=0.010mol](/tpl/images/0030/3484/34688.png)
![[I_2] _{eq} =(0.006M -0.00484M)*65.1L=0.078mol](/tpl/images/0030/3484/28b57.png)


