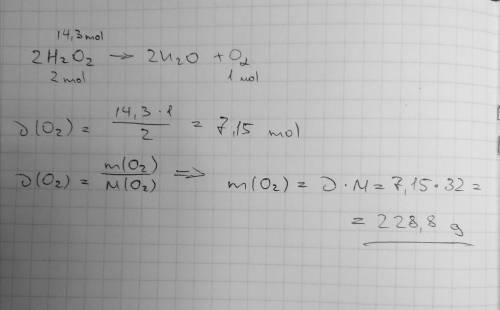Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Mm h2o2 = 34.02 g/mol mm h2o = 18.02 g/mol mm o2 = 32 g/mol 2h2o2 —> 2h2o + o2 if 14.3 moles of...
Questions


Biology, 04.08.2019 02:00

Mathematics, 04.08.2019 02:00




Business, 04.08.2019 02:00

Social Studies, 04.08.2019 02:00

Computers and Technology, 04.08.2019 02:00

Geography, 04.08.2019 02:00


Biology, 04.08.2019 02:00

Social Studies, 04.08.2019 02:00

Chemistry, 04.08.2019 02:00

Mathematics, 04.08.2019 02:00

Mathematics, 04.08.2019 02:00

Mathematics, 04.08.2019 02:00