
Chemistry, 29.06.2019 05:00 natishtaylor1p8dirz
An atom of sodium-23 (na-23) has a net charge of +1. identify the number of protons, neutrons, and electrons in the atom. then, explain how you determined the number of each type of particle.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
An atom of sodium-23 (na-23) has a net charge of +1. identify the number of protons, neutrons, and e...
Questions

Mathematics, 21.05.2020 05:09

Mathematics, 21.05.2020 05:09



English, 21.05.2020 05:09

Geography, 21.05.2020 05:09

English, 21.05.2020 05:09

Mathematics, 21.05.2020 05:09




History, 21.05.2020 05:09



Mathematics, 21.05.2020 05:09


History, 21.05.2020 05:09


Mathematics, 21.05.2020 05:09

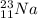
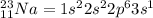
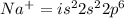 (It will loose one electron)
(It will loose one electron)

