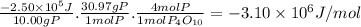Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1
You know the right answer?
When 10.00 g of phosphorus is burned in o2(g) to form p4o10(s), enough heat is generated to raise th...
Questions

Biology, 12.02.2020 03:15


Mathematics, 12.02.2020 03:15

Mathematics, 12.02.2020 03:15


Mathematics, 12.02.2020 03:15







Mathematics, 12.02.2020 03:15