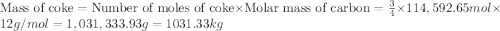The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s)→ 4fe(s)+ 3o2(g) is driven by the high-temperature combustion of coke, a purified form of coal: c(s)+ o2(g)→ co2(g) suppose at the temperature of a blast furnace the gibbs free energies of formation δgf of co2 and fe2o3 are −429./kjmol and −835./kjmol, respectively. calculate the minimum mass of coke needed to produce 6400.kg of pure iron. round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s...
Questions

Biology, 09.11.2020 17:30

Biology, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30



Mathematics, 09.11.2020 17:30



Mathematics, 09.11.2020 17:30



Advanced Placement (AP), 09.11.2020 17:30



Mathematics, 09.11.2020 17:30


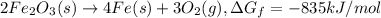 ...(1)
...(1)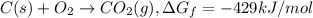 ...(2)
...(2)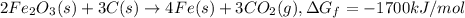 ...(3)
...(3)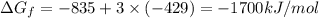
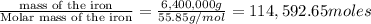
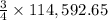 moles of coke.
moles of coke.