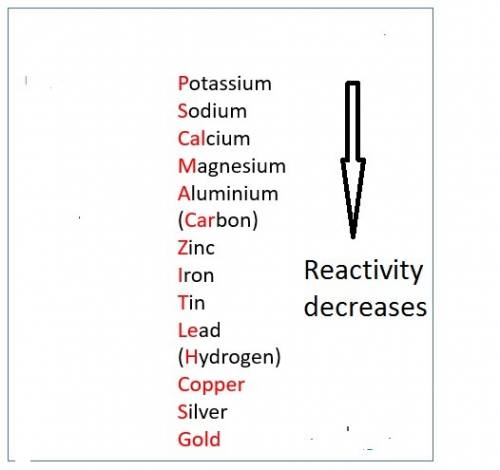
When hno3 and au are mixed together, what do you expect to happen, and why? a. no reaction, because au is above h on the activity series and they cannot react b. no reaction, because au is lower on the activity series and cannot replace h c. they form au(no3)2 and h2, because au is more reactive and able to replace h d. they form au(no3)2 and h2, because they trade places in a double replacement reaction

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

You know the right answer?
When hno3 and au are mixed together, what do you expect to happen, and why? a. no reaction, because...
Questions

Mathematics, 17.01.2020 06:31

Mathematics, 17.01.2020 06:31

Mathematics, 17.01.2020 06:31



History, 17.01.2020 06:31

Computers and Technology, 17.01.2020 06:31

Mathematics, 17.01.2020 06:31


Mathematics, 17.01.2020 06:31






Computers and Technology, 17.01.2020 06:31




Mathematics, 17.01.2020 06:31

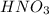 . Thus there will be no reaction.
. Thus there will be no reaction.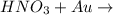 no reaction
no reaction