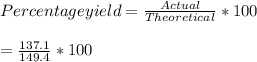Chemistry, 29.06.2019 02:00 101EXPERIENCE
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reagent, 8.3 mol of h2s were consumed, and 137.1 g of water were collected after the reaction has gone to completion, what is the percent yield of the reaction? show your work.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reag...
Questions



Advanced Placement (AP), 11.11.2020 06:10

Mathematics, 11.11.2020 06:10

Mathematics, 11.11.2020 06:10




Social Studies, 11.11.2020 06:10

English, 11.11.2020 06:10

Mathematics, 11.11.2020 06:10

Mathematics, 11.11.2020 06:10


Social Studies, 11.11.2020 06:10


Chemistry, 11.11.2020 06:10

History, 11.11.2020 06:10


Physics, 11.11.2020 06:10