
Chemistry, 28.06.2019 22:00 randallmatthew6124
Amixture of reactants and products has a equilibrium constant of 4.7 and initially contains 0.035 m h2o, 0.050 m ch4, 0.15 m co, and 0.20 m h2. in which direction will the reaction go in order to attain equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
Amixture of reactants and products has a equilibrium constant of 4.7 and initially contains 0.035 m...
Questions

Mathematics, 09.10.2019 05:30


English, 09.10.2019 05:30


Biology, 09.10.2019 05:30

Mathematics, 09.10.2019 05:30



Physics, 09.10.2019 05:30

Health, 09.10.2019 05:30

English, 09.10.2019 05:30


Mathematics, 09.10.2019 05:30

Mathematics, 09.10.2019 05:30

Mathematics, 09.10.2019 05:30





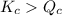 reaction will move in forward direction.
reaction will move in forward direction.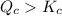 reaction will move in backward direction.
reaction will move in backward direction.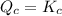 reaction is at equilibrium.
reaction is at equilibrium.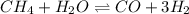
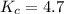
![[H_2O]=0.035 M,[CH_4]=0.050 M,[CO]=0.15 M,[H_2]=0.20 M](/tpl/images/0028/6761/d78ff.png)
![Q_{c}=\frac{[CO][H_2]^3}{[H_2O][CH_4]}=\frac{0.15\times (0.20)^3}{0.035\times 0.050}=0.6857](/tpl/images/0028/6761/f91f9.png)


