Chemistry, 28.06.2019 22:00 sarbjit879
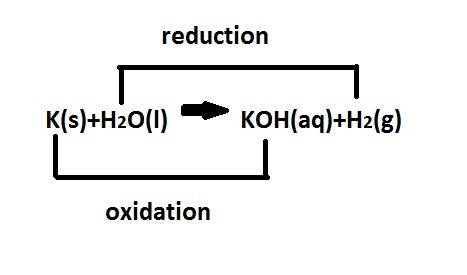
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2k(s)+2h2o=2koh(aq)+h2(g). your lab partner says this is a redox reaction and a combustion reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide...
Questions


English, 26.06.2019 02:30



Chemistry, 26.06.2019 02:30




Spanish, 26.06.2019 02:30




History, 26.06.2019 02:30

French, 26.06.2019 02:30

Mathematics, 26.06.2019 02:30

History, 26.06.2019 02:30

Mathematics, 26.06.2019 02:30



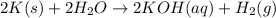
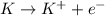 oxidation
oxidation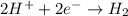 reduction
reduction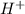 ions of water by gaining electrons (from potassium in water) gives hydrogen gas.
ions of water by gaining electrons (from potassium in water) gives hydrogen gas.