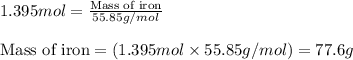Chemistry, 28.06.2019 20:30 Wondersixeleven
When 125 grams of feo react with 25.0 grams of al, how many grams of fe can be produced? feo + al → fe + al2o3 25.9 g fe 38.7 g fe 50.9 g fe 77.6 g fe

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
When 125 grams of feo react with 25.0 grams of al, how many grams of fe can be produced? feo + al →...
Questions





English, 20.09.2020 09:01

English, 20.09.2020 09:01

English, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01


Chemistry, 20.09.2020 09:01

English, 20.09.2020 09:01

English, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Law, 20.09.2020 09:01


Social Studies, 20.09.2020 09:01

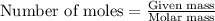 .....(1)
.....(1)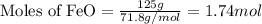
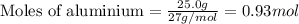
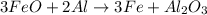
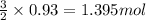 of FeO
of FeO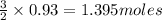 of iron metal
of iron metal