
Chemistry, 28.06.2019 20:00 AlexGaming1822
If you make a solution by dissolving 1.0 mol of fecl3 into 1.0 kg of water, how would the osmotic pressure of this solution compare with the osmotic pressure of a solution that is made from 1.0 mol of glucose in 1.0 kg of water? one-half as large the same twice as large four times as large

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

You know the right answer?
If you make a solution by dissolving 1.0 mol of fecl3 into 1.0 kg of water, how would the osmotic pr...
Questions


Physics, 02.12.2019 04:31


Mathematics, 02.12.2019 04:31


Social Studies, 02.12.2019 04:31




English, 02.12.2019 04:31


History, 02.12.2019 04:31

Mathematics, 02.12.2019 04:31


Mathematics, 02.12.2019 04:31

Arts, 02.12.2019 04:31


Chemistry, 02.12.2019 04:31


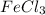 in 1.0 kg water and 1.0 mol glucose in 1.0 kg water.
in 1.0 kg water and 1.0 mol glucose in 1.0 kg water. 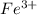 and 3
and 3 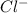 ions.
ions.

