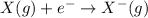Chemistry, 28.06.2019 18:30 nayelimoormann
Elaborate on how electron affinity and ion formation relate. a) electron affinity is the energy required to add one electron to an atom forming an anion. b) electron affinity is the energy required to remove one electron from an atom forming a cation. c) electron affinity is the energy produced when adding an electron to an atom forming an isotope. d) electron affinity is the energy donated to discharge one electron from an atom forming a baryon.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Elaborate on how electron affinity and ion formation relate. a) electron affinity is the energy requ...
Questions

Mathematics, 20.10.2020 02:01

Advanced Placement (AP), 20.10.2020 02:01



Mathematics, 20.10.2020 02:01

Computers and Technology, 20.10.2020 02:01


English, 20.10.2020 02:01


Chemistry, 20.10.2020 02:01





Mathematics, 20.10.2020 02:01


Chemistry, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01