
In this reaction, what roll does the lead (ii) nitrate play when 50.0 ml of 0.100m iron (iii) chloride are mixed with 50.0 ml of 0.100m lead (ii) nitrate? a) lead (ii) nitrate increases the amount of precipitate. b) the reactant lead (ii) nitrate decreases product yield. c) lead (ii) nitrate is the excess reactant in the reaction. d) the lead (ii) nitrate is the reaction's limiting reactant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
In this reaction, what roll does the lead (ii) nitrate play when 50.0 ml of 0.100m iron (iii) chlori...
Questions




















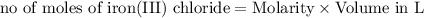
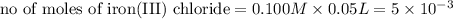
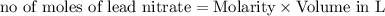
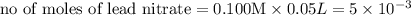
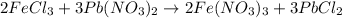
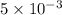 moles of lead nitrate react with
moles of lead nitrate react with 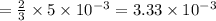 of ferric chloride.
of ferric chloride.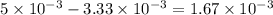 moles of ferric chloride will be left unreacted.
moles of ferric chloride will be left unreacted.

