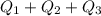Chemistry, 28.06.2019 18:00 nuconteaza119
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions

Mathematics, 13.03.2021 05:00

Mathematics, 13.03.2021 05:00

Mathematics, 13.03.2021 05:00

History, 13.03.2021 05:00






English, 13.03.2021 05:00

English, 13.03.2021 05:00






English, 13.03.2021 05:00



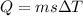
 is the change in temperature.
is the change in temperature.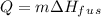
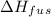 is the enthalpy of fusion.
is the enthalpy of fusion.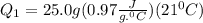
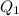 = 509.25 J
= 509.25 J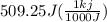

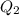 = 2.72 kj
= 2.72 kj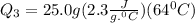
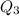 = 3680 J
= 3680 J