
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1


Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

You know the right answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions


Mathematics, 22.04.2020 05:25

English, 22.04.2020 05:25



Biology, 22.04.2020 05:25




English, 22.04.2020 05:25




Chemistry, 22.04.2020 05:25

Mathematics, 22.04.2020 05:25

Mathematics, 22.04.2020 05:25

Mathematics, 22.04.2020 05:25

Social Studies, 22.04.2020 05:25

Mathematics, 22.04.2020 05:25

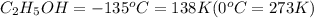
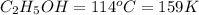
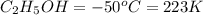
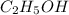 at 159 K
at 159 K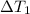 = 159 K - 138 K = 21 K
= 159 K - 138 K = 21 K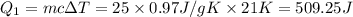
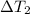 = 223 K - 159 K = 64 K
= 223 K - 159 K = 64 K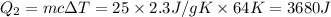
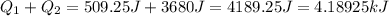 (1kJ=1000J)
(1kJ=1000J)


