Chemistry, 28.06.2019 18:00 itscheesycheedar
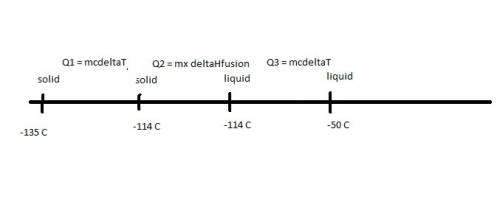
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1
You know the right answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions




Mathematics, 05.01.2021 19:50


English, 05.01.2021 19:50

Social Studies, 05.01.2021 19:50




Mathematics, 05.01.2021 19:50


Mathematics, 05.01.2021 19:50

Mathematics, 05.01.2021 19:50

Biology, 05.01.2021 19:50


Mathematics, 05.01.2021 19:50

English, 05.01.2021 19:50


History, 05.01.2021 19:50