
Chemistry, 28.06.2019 18:00 Lacrosse34
The hydrides of group 5a are nh3, ph3, ash3, and sbh3. arrange them from highest to lowest boiling point. rank the molecules from highest to lowest boiling point. to rank items as equivalent, overlap them.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3


Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
The hydrides of group 5a are nh3, ph3, ash3, and sbh3. arrange them from highest to lowest boiling p...
Questions

English, 24.10.2020 06:00

Physics, 24.10.2020 06:00

Mathematics, 24.10.2020 06:00


Chemistry, 24.10.2020 06:00


Social Studies, 24.10.2020 06:00

Mathematics, 24.10.2020 06:00

Arts, 24.10.2020 06:00

Mathematics, 24.10.2020 06:00



Mathematics, 24.10.2020 06:00

History, 24.10.2020 06:00



SAT, 24.10.2020 06:00


English, 24.10.2020 06:00

English, 24.10.2020 06:00

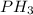 to
to 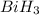 . But boiling point of
. But boiling point of 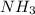 is higher than rest of the hydrides of group 5A.
is higher than rest of the hydrides of group 5A. 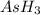 but lower than
but lower than 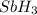 and
and 

