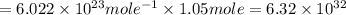Chemistry, 28.06.2019 14:00 spiderman66
Solid sodium reacts violently with water, producing heat, hydrogen gas, and sodium hydroxide. how many molecules of hydrogen gas are formed when 48.7 g of sodium are added to water? show your work. (4 points) 2na + 2h2o > 2naoh + h2 2na + 2h2o > 2naoh + h2 48.7 g ? molecules 2 mol na= 1 mol h2 1 mol na= 23g na 1 mol h2= 6.02x1023 molecules (48.7g na/1)x(1 mol na/23g na)x(1 mol h2/2mol na)x(6.02x1023/1 mol h2) (48.7)(6.02x1023)/(23)(2)= 293.174x1023 molecules can someone tell me what i did wrong?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
Solid sodium reacts violently with water, producing heat, hydrogen gas, and sodium hydroxide. how ma...
Questions

Mathematics, 14.11.2019 00:31


Mathematics, 14.11.2019 00:31

Physics, 14.11.2019 00:31

Social Studies, 14.11.2019 00:31


Social Studies, 14.11.2019 00:31


Physics, 14.11.2019 00:31









Mathematics, 14.11.2019 00:31


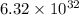
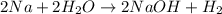
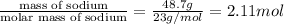
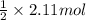 of hydrogen gas that is 1.05 moles of hydrogen gas.
of hydrogen gas that is 1.05 moles of hydrogen gas.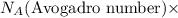 moles of substance
moles of substance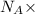 moles of hydrogen gas
moles of hydrogen gas