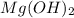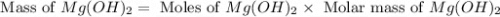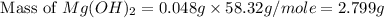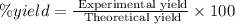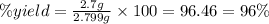Select the correct answer. excess sodium hydroxide is added to a solution containing 4.6 grams of magnesium chloride. a reaction takes place according to this equation: 2naoh(aq) + mgcl2(aq) → 2nacl(aq) + mg(oh)2(s). the magnesium hydroxide produced by the reaction was collected and weighed. if the mass of the magnesium hydroxide was 2.7 grams, what was the percent yield? use the periodic table. a. 48% b. 59% c. 61% d. 96%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Select the correct answer. excess sodium hydroxide is added to a solution containing 4.6 grams of ma...
Questions



Business, 28.07.2019 07:20

Biology, 28.07.2019 07:20

Business, 28.07.2019 07:20

History, 28.07.2019 07:20

Biology, 28.07.2019 07:20

Arts, 28.07.2019 07:20











Social Studies, 28.07.2019 07:20

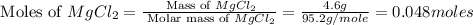
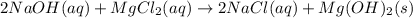
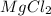 react to give 1 mole of
react to give 1 mole of