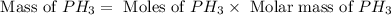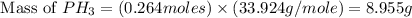Chemistry, 28.06.2019 11:00 kaylarenee05080
12.use the equation below to determine the maximum number of grams of ph3 that can be formed when 8.2 g of phosphorus reacts with 4.0 g of hydrogen to form ph3? note that the molar mass of phosphorus is 30.9 g/ mol and hydrogen is 1.008 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
12.use the equation below to determine the maximum number of grams of ph3 that can be formed when 8....
Questions

History, 04.07.2019 16:00


Mathematics, 04.07.2019 16:00

Computers and Technology, 04.07.2019 16:00

Spanish, 04.07.2019 16:00





Chemistry, 04.07.2019 16:00




Chemistry, 04.07.2019 16:00





Chemistry, 04.07.2019 16:00

Mathematics, 04.07.2019 16:00

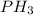 formed is, 8.955 g
formed is, 8.955 g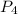 = 123.6 g/mole
= 123.6 g/mole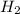 = 2.016 g/mole
= 2.016 g/mole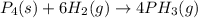
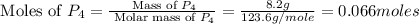
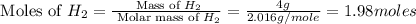
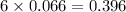 moles of
moles of 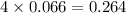 moles of
moles of