
Chemistry, 28.06.2019 09:00 Zachary4759
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specific heats of ice, water, and steam are 2.09 j/g−k, 4.18 j/g−k, and 1.84 j/g−k, respectively. for h2o, δ hfus = 6.01kj/mol, and δhvap = 40.67 kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

You know the right answer?
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specif...
Questions

Physics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31





Mathematics, 30.12.2019 20:31

History, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

History, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

History, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

History, 30.12.2019 20:31

Biology, 30.12.2019 20:31


Biology, 30.12.2019 20:31



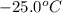 to water at
to water at 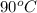 is, 7.712 KJ
is, 7.712 KJ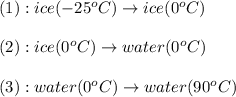
![\Delta H=[m\times c_{ice}\times (T_2-T_1)]+\Delta H_{fusion}+[m\times c_{water}\times (T_3-T_2)]](/tpl/images/0026/6160/eeaad.png)
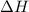 = enthalpy change
= enthalpy change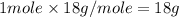
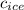 = specific heat of ice = 2.09 J/gk
= specific heat of ice = 2.09 J/gk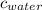 = specific heat of water = 4.18 J/gk
= specific heat of water = 4.18 J/gk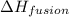 = enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole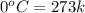
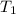 = initial temperature of ice =
= initial temperature of ice = 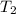 = final temperature of ice =
= final temperature of ice = 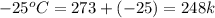
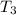 = initial temperature of water =
= initial temperature of water = 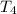 = final temperature of water =
= final temperature of water = 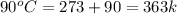
![\Delta H=[18g\times 2.09J/gK\times (273-248)k]+0.00601J+[18g\times 4.18J/gK\times (363-273)k]](/tpl/images/0026/6160/21744.png)
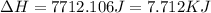 (1 KJ = 1000 J)
(1 KJ = 1000 J)

