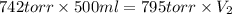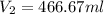Chemistry, 28.06.2019 09:00 KingOfLaBron23
Acontainer holds 500. ml of co2 at 20.° c and 742 torr. what will be the volume of the co2 if the pressure is increased to 795 torr?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Acontainer holds 500. ml of co2 at 20.° c and 742 torr. what will be the volume of the co2 if the pr...
Questions





Mathematics, 22.02.2021 18:50



English, 22.02.2021 18:50

Mathematics, 22.02.2021 18:50

Mathematics, 22.02.2021 18:50

English, 22.02.2021 18:50


Mathematics, 22.02.2021 18:50

Spanish, 22.02.2021 18:50



Physics, 22.02.2021 18:50

History, 22.02.2021 18:50


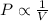 (At constant temperature and number of moles)
(At constant temperature and number of moles)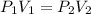 [according to Boyle's law]
[according to Boyle's law]