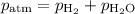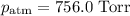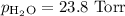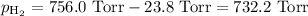Chemistry, 28.06.2019 07:30 emmaty7845
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr and the temperature is 25 °c. the vapor pressure of water at various temperatures can be found in this table. calculate the molar mass of the metal.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291...
Questions


Physics, 14.01.2021 21:40



History, 14.01.2021 21:40

Mathematics, 14.01.2021 21:40

English, 14.01.2021 21:40

History, 14.01.2021 21:40

Chemistry, 14.01.2021 21:40

Chemistry, 14.01.2021 21:40


Mathematics, 14.01.2021 21:40

English, 14.01.2021 21:40

English, 14.01.2021 21:40

Chemistry, 14.01.2021 21:40



Social Studies, 14.01.2021 21:40