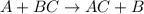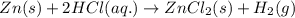Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because zinc is more reactive than hydrogen. a single replacement reaction takes place because zinc is more reactive than chlorine. a double replacement reaction takes place because zinc is less reactive than hydrogen. a double replacement reaction takes place because zinc is less reactive than chlorine.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1



Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this rea...
Questions


Mathematics, 10.05.2021 21:50

Social Studies, 10.05.2021 21:50


Mathematics, 10.05.2021 21:50


Health, 10.05.2021 21:50





Mathematics, 10.05.2021 21:50



Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50

Mathematics, 10.05.2021 21:50



Mathematics, 10.05.2021 21:50